Peking University, February 16, 2025: Professor Ma Ding’s research team from the College of Chemistry and Molecular Engineering at Peking University, along with collaborators, has made significant strides in hydrogen production. On February 13 and 14, 2025, they published two papers in Nature and Science, titled “Shielding Pt/γ-Mo2N by inert nano-overlays enables stable H2 production” and “Thermal catalytic reforming for hydrogen production with zero CO2 emission” respectively, pushing the boundaries of hydrogen production efficiency via different approaches.
Why it matters:
Hydrogen energy plays a pivotal role in the global transition to carbon neutrality. Achieving high efficiency, stability, and cost-effectiveness in hydrogen production is crucial for transforming the energy landscape.
1.Traditional catalysts like Pt and Au on molybdenum carbide (α-MoC) are highly active but suffer from rapid deactivation in methanol-water reforming (MSR) due to oxidation, hindering their industrial application.
2.Bioethanol is a promising hydrogen source e. However, traditional hydrogen production from ethanol-water reforming faces two major problems: first, high temperatures (400-600°C) result in high energy consumption and CO₂ emissions; second, existing catalysts are prone to carbon deposition and sintering, limiting their industrial application by reducing efficiency and durability.
What’s new:
1.The Nature paper, titled “Shielding Pt/γ-Mo2N by inert nano-overlays enables stable H2 production”, focuses on enhancing catalyst stability. The team introduces a rare-earth element-modified catalyst. By adding an inert rare-earth oxide (e.g., La₂O₃) nano-overlay on Pt/γ-Mo₂N, the team enhances the catalyst’s stability, significantly prolonging its lifetime and ensuring long-term hydrogen production stability.
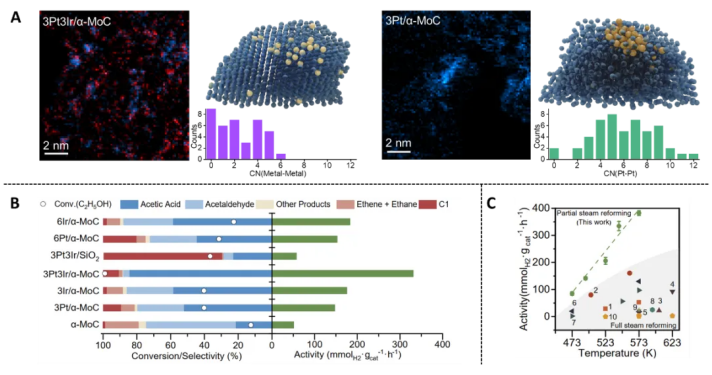
2.The Science paper, titled “Thermal catalytic reforming for hydrogen production with zero CO2 emission”, presents a new method for zero-carbon hydrogen production from ethanol reforming. In collaboration with Zhou Jihan’s team, the researchers use a Pt-Ir/α-MoC interface catalyst to avoid ethanol C-C bond cleavage, operating at 270°C to yield high hydrogen output while co-producing acetic acid and eliminating CO₂ emissions. This technique supports a carbon-neutral circular economy, integrating hydrogen and chemical co-production.
Key findings:
1.The Pt/La-Mo₂N catalyst maintains stability for over 1000 hours in methanol reforming, with no significant deactivation. Achieving a catalytic turnover number (TON) exceeding 15 million, it sets a new benchmark for methanol-water hydrogen production. This innovation can be expanded to other rare-earth elements (La, Y, Pr, Ho) and even non-rare-earth elements (Ca, Sr).
Pt/La-Mo₂N catalytic structure and performance in hydrogen production
2.The Pt-Ir/α-MoC catalyst not only generates hydrogen but also produces acetic acid with near-zero CO₂ emissions. Under 270°C, it achieves a hydrogen production rate of 331.3 mmol/g·hr and an acetic acid selectivity of 84.5%. After 100 hours, the catalyst shows excellent resistance to deactivation. This process reduces carbon emissions by 62% compared to traditional methods for acetic acid production, forming a “hydrogen production - carbon storage - acid yield” closed-loop system.
Pt-Ir/α-MoC catalytic structure and performance
Significance:
1.Catalyst performance: The Nature paper’s breakthrough in stabilizing Pt/γ-Mo₂N catalysts addresses a critical bottleneck in the design of high-stability interface catalysts without compromising activity, enabling cost-effective, durable applications in green energy, hydrogen fuel cells, and sustainable chemical industries.
2.Zero CO2 emission: The Science paper introduces a transformative approach to hydrogen production and carbon storage, poised to advance the global transition to a low-carbon economy and support global carbon neutrality goals.
3.Technological complementation: The rare-earth-modified catalysts improve hydrogen production efficiency and lifetime, making large-scale industrial hydrogen production feasible. The zero-CO₂ emission process exemplifies a green chemistry approach to resource-efficient hydrogen-chemical co-production.
Links to the papers:
https://www.nature.com/articles/s41586-024-08483-w
https://www.science.org/doi/10.1126/science.adt0682
Written by: He Yike
Edited by: Zhang Jiang
Source: PKU Wechat (Chinese)