
Li Mo is a professor at PKU Third Hospital
Peking University, May 16, 2025: A research team led by Professor Li Mo at Peking University Third Hospital, in collaboration with Professor Lingqian Chang's team from Beihang University, Professor Cunjiang Yu's team from the University of Illinois Urbana-Champaign, and Professor Xinge Yu’s team from City University of Hong Kong, has developed a revolutionary battery-free, flexible NanoFLUID patch for precise drug and gene delivery to internal organs. Their study, titled "A battery-free nanofluidic intracellular delivery patch for internal organs," was published in Nature, marking a breakthrough that overcomes key biological barriers. The technology enables highly efficient, low-toxicity intracellular therapy with single-cell precision, integrating medicine, engineering, and chemistry to advance targeted treatments for severe diseases.
Why It Matters
Targeted delivery of therapeutic agents to specific organs faces three major challenges: First, conventional methods show extremely low efficiency, with less than 2% of nanocarriers reaching target tissues due to rapid clearance by the liver and kidneys. Second, poor distribution kinetics lead to significant off-target effects. Third, physiological barriers including vascular endothelium, organ-specific microenvironments, and cell membranes prevent effective delivery to deep organs like the pancreas, heart, and liver. While viral vectors offer better transfection efficiency, their potential immunogenicity and genomic integration risks limit clinical applications.
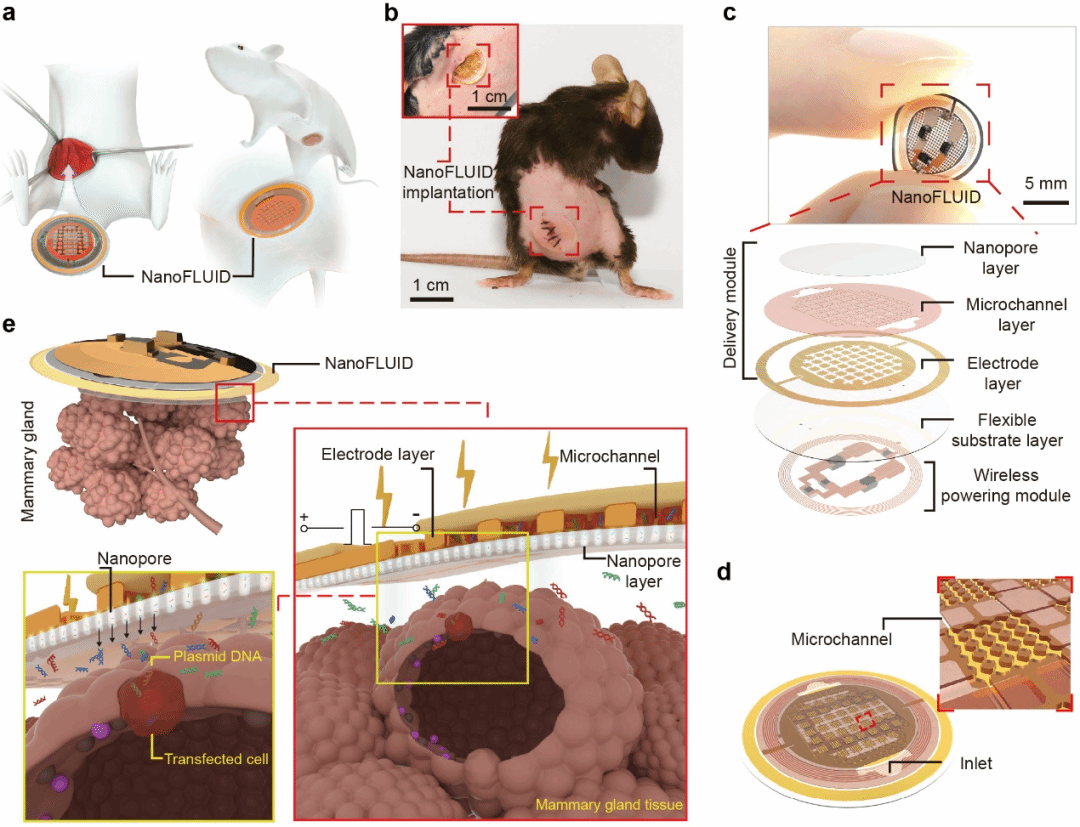
Fig. 1. Design and Working Principle of NanoFLUID.
NanoFLUID Patch Innovation
The patch's innovative design features three key elements: (1) a battery-free, chip-free flexible structure that matches the mechanical properties of soft tissues; (2) a "nano-pore–microchannel–microelectrode" system enabling precise control; and (3) low-voltage (10V) operation for safe electroporation.
In validation studies, the technology demonstrated delivery efficiency improvements of several orders of magnitude compared to diffusion-based methods, while maintaining cell viability above 95% and achieving single-cell precision. This combination of features creates a programmable platform that overcomes both physical and biological barriers to organ-targeted therapy.
Key findings
In acute liver injury models, the NanoFLUID patch significantly improved delivery efficiency of therapeutic molecules, accelerating tissue regeneration. For breast tumor models, the technology enabled precise modeling with high efficacy and minimal toxicity.
The research team further leveraged NanoFLUID's capabilities to co-transfect gene libraries, leading to the identification of DUS2, a lung-specific metastasis driver gene. Through high-throughput sequencing and bioinformatics analysis, they discovered that DUS2 enhances translation of over 70 metastasis-related proteins, promoting tumor cell colonization in lungs.
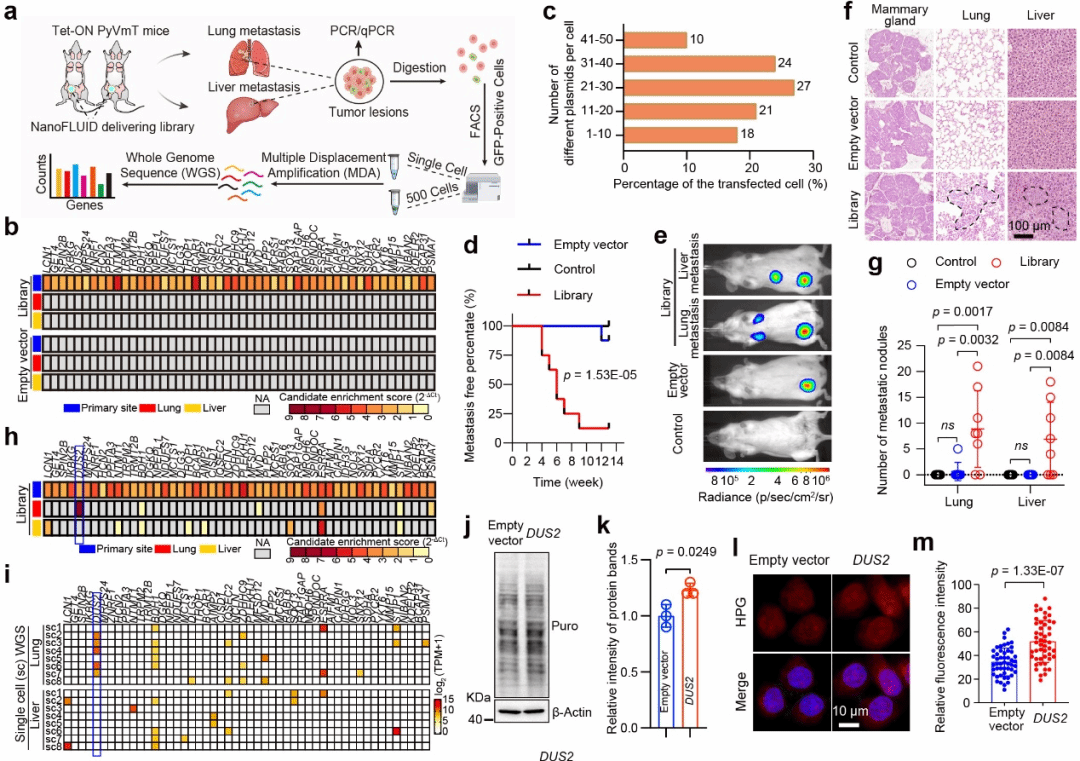
Fig. 2. NanoFLUID-Based Screening of Tumor Metastasis Drivers and Targeted Therapy.
Significance
The NanoFLUID technology represents a paradigm shift in targeted therapy, offering unprecedented control and precision. Beyond its immediate therapeutic applications, the platform enables fundamental biological discoveries and paves the way for personalized treatment approaches. This advancement could transform how we treat chronic diseases, from cancer to organ failure.
*This article is featured in the "Why It Matters" series. More from this series.
Click “
here” to read the paper.
Written by: Akaash Babar
Edited by: Zhang Jiang
Source: PKU News (
Chinese)


