Peking University, July 07, 2025: A research team led by Prof. Pan Feng from the School of Advanced Materials, Peking University Shenzhen Graduate School has uncovered key mechanisms that govern how protons are stored and transported in aqueous batteries. The study provides critical insights that could lead to safer, faster-charging, and higher-capacity alternatives to today’s lithium-ion batteries. Published in Matter, a Cell Press journal, the study titled “Proton storage and transfer in aqueous batteries” reveals how hydrogen-bond network engineering enables efficient proton storage and transport.
Background
Aqueous batteries, which use water-based electrolytes, are inherently safer than lithium-ion systems but have traditionally suffered from lower energy density. Protons, due to their low mass and high mobility, hold great promise, but their complex chemistry has limited real-world application.
Pan’s team demonstrates that protons move through a Grotthuss-type mechanism, hopping between hydrogen bonds rather than diffusing like metal ions. This allows for ultra-fast, “diffusion-free” transport and positions protons as ideal charge carriers for high-performance aqueous batteries.
Why it matters
This research addresses a longstanding challenge in energy storage: achieving both safety and high performance. By revealing how hydrogen-bond networks facilitate proton storage and transport, the study lays a solid theoretical foundation for a new generation of energy systems that could match or exceed lithium-ion technology. Unlike lithium (Li⁺) and sodium (Na⁺), which form stable ionic bonds with oxygen in rigid crystal frameworks, protons (H⁺) form more covalent, saturable H–O bonds and do not integrate into lattices in the same way.
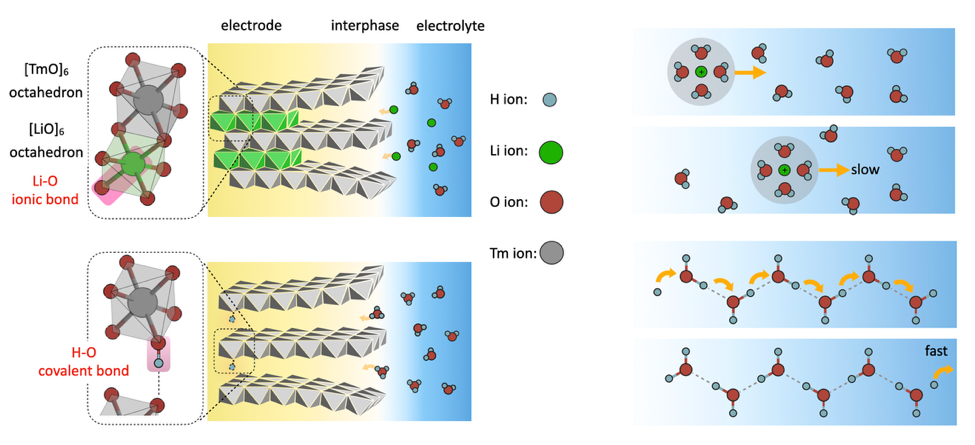
Figure 1. The bonding, storage, and transport of protons differ from those of other metal cations.
Key Findings
A significant contribution of the study is the proposal of three core strategies to optimize aqueous battery performance using hydrogen-bond network engineering.
First, in electrode design, the researchers suggest embedding water-containing or anhydrous hydrogen-bond networks within solid-state materials to create well-defined pathways for proton transport. Second, through electrolyte tuning, they demonstrate that adjusting the concentration of acids and the type of anions present in the electrolyte can stabilize and enhance proton conductivity. Third, in terms of interface engineering, the team shows that modifying the electrode surface, such as by introducing hydroxyl (–OH) and carboxyl (–COOH) groups using oxygen plasma treatment, can create proton-bridging channels that significantly lower interfacial charge-transfer resistance and improve reaction kinetics.
Together, these strategies form a unified framework that clarifies proton behavior in aqueous systems and paves the way for safer, faster, and more efficient energy storage.
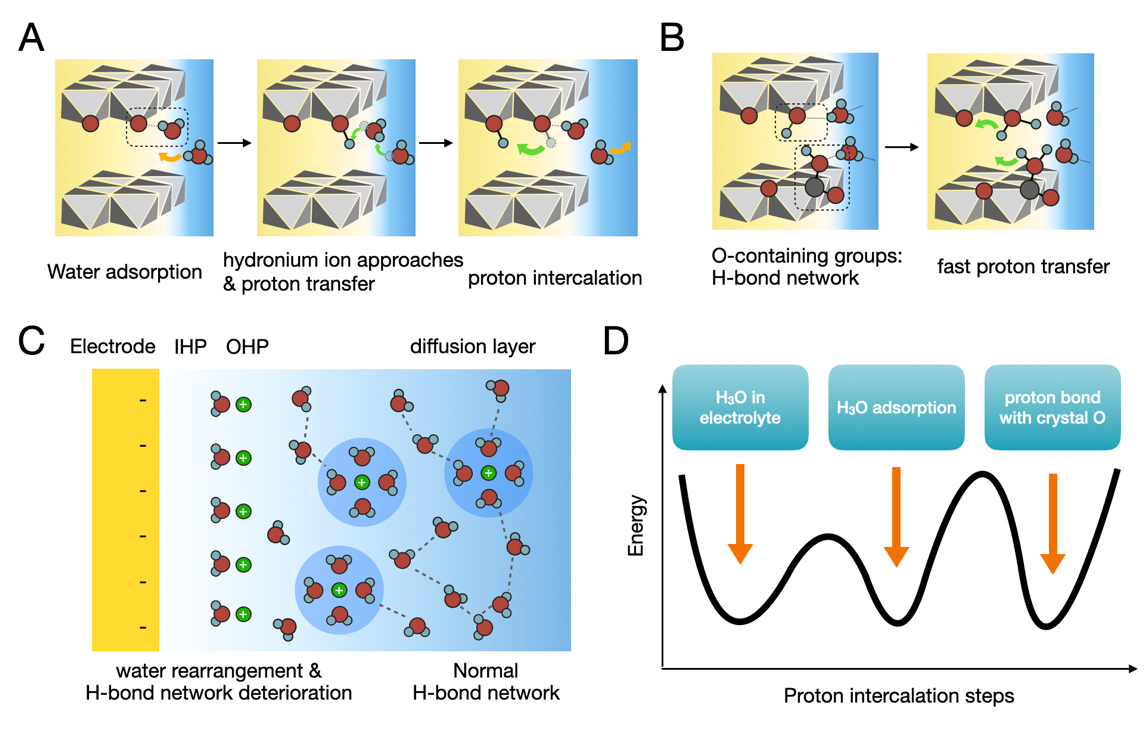
Figure 2. Proton transport characteristics at the electrode/electrolyte interface.
Future Implications
This study paves the way for next-generation proton-based aqueous batteries that combine safety with high performance. By engineering hydrogen-bond networks, future devices could achieve higher energy density, faster charging, and longer lifespan, advancing applications from grid storage to portable electronics and electric vehicles.
*This article is featured in PKU News "Why It Matters" series. More from this series.
Read more: https://doi.org/10.1016/j.matt.2025.102165
Written by: Akaash Babar
Edited by: Zhang Jiang
Source: PKU News (
Chinese)

