Peking University, July 23, 2025: On July 14, 2025, Professor Su Xiaodong’s team from the School of Life Sciences at Peking University, in collaboration with Professor John Rasko’s team from the University of Sydney, published a groundbreaking study in Cell titled “An alternate receptor for adeno-associated viruses”. The paper reports the discovery of a previously unknown receptor for adeno-associated viruses (AAVs): Carboxypeptidase D (CPD), now named AAVR2.
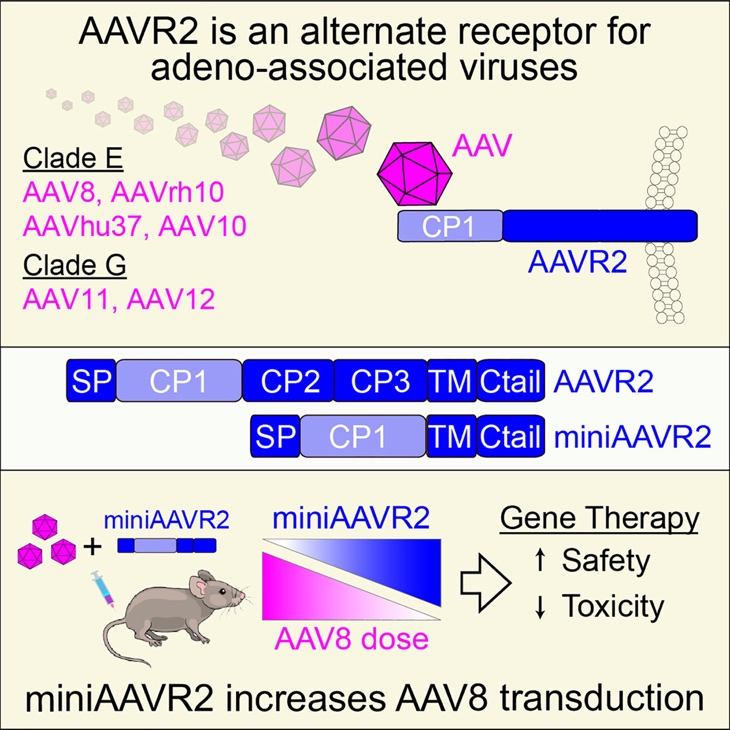
Figure 1. This study reveals that Carboxypeptidase D (CPD) functions as a high-affinity receptor for Clade E and G AAVs and has been designated AAVR2.
Background
Adeno-associated viruses (AAVs) have been approved in many countries as gene therapy vectors for treating a range of genetic disorders, including retinitis pigmentosa, spinal muscular atrophy, Duchenne muscular dystrophy, and hemophilia. These clinical successes are largely due to AAV’s ability to transduce human tissues both safely and efficiently, making it the most widely used in clinical gene therapy.
Why It Matters
This study uncovers a new receptor-mediated transduction pathway for AAVs. It shows that AAVR2 enables efficient gene delivery independently of the traditional AAVR receptor, depending on the viral serotype. The discovery not only advances our understanding of AAV biology but also offers new opportunities to enhance gene therapy through receptor engineering and tissue-specific targeting.
Key finding
The Sydney research team constructed a whole-genome CRISPR/SAM transcriptional activation library and introduced it into AAVR-knockout cell lines. They then transduced these cells with AAV8-EGFP and enriched highly infected cells using flow cytometry. Subsequent NGS analysis successfully identified CPD as a key gene involved in AAV infection. Further validation experiments confirmed that AAVR2 mediates the transduction of Clade E AAV serotypes (including AAV8, AAVrh10, and AAVhu37) as well as naturally occurring AAV11 and AAV12, all independently of AAVR.
To understand the structural basis of this interaction, researchers at Peking University overexpressed and purified the full extracellular domain and several fragments of AAVR2 in HEK293 cells. ELISA and surface plasmon resonance (SPR) assays demonstrated a strong direct interaction between AAVR2 and the AAV8 capsid, primarily mediated by AAVR2’s first domain, CP1. The dissociation constant reached KD = 73.8 nM. Using cryo-electron microscopy (cryo-EM), the PKU team resolved the high-resolution structure of the AAV8-CP1 complex. They found that CP1 binds at the 2-fold axis of the AAV8 capsid and is surrounded by several variable loops (VR-loops). Among these, the VR-VIII loop played a key role, directly interacting with CP1. Remarkably, grafting this loop from AAV8 into AAV2 enabled AAV2 to acquire AAVR2-dependent transduction ability, a direct demonstration of how this region determines receptor specificity.
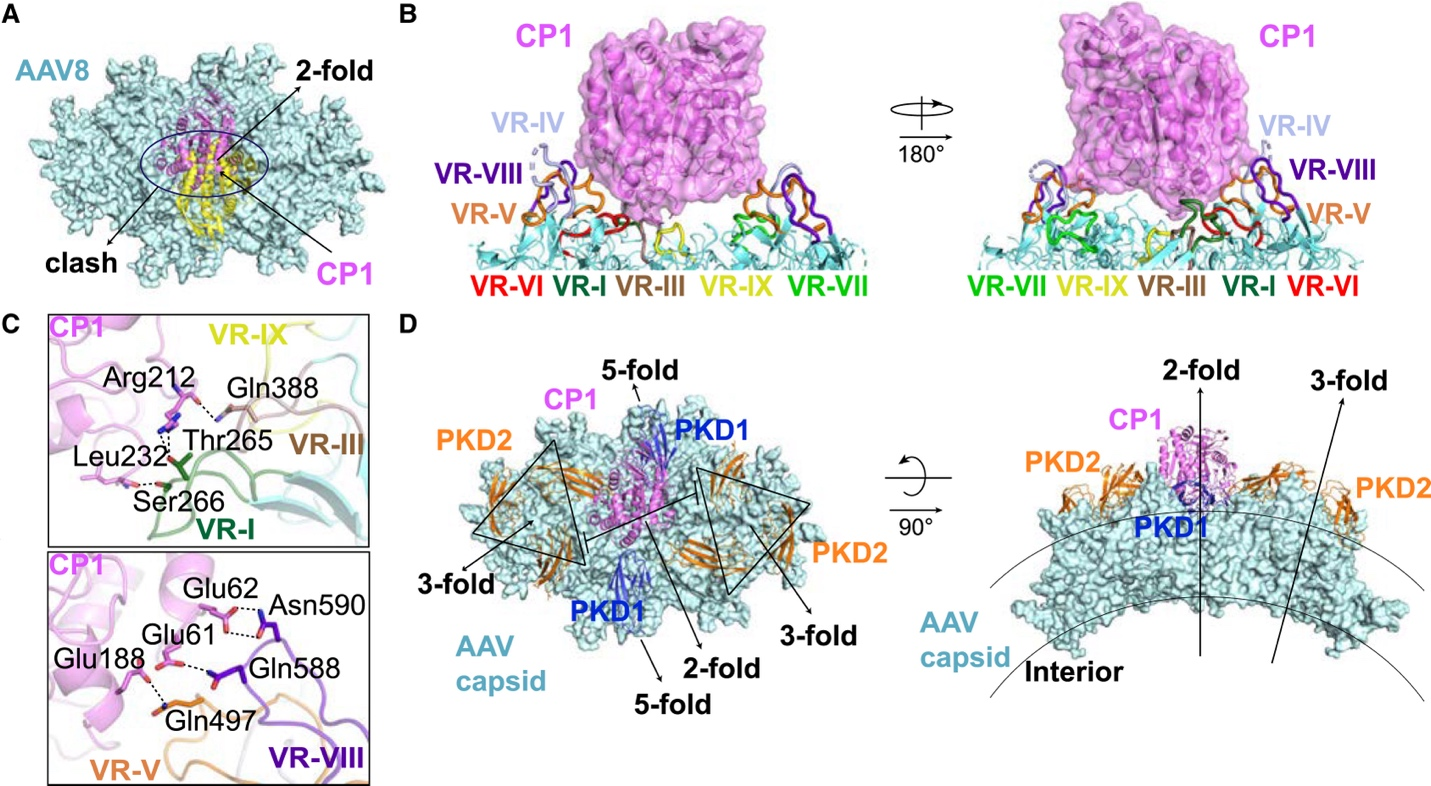
Figure 2. The first domain of AAVR2 (CPD), CP1, binds to the 2-fold axis region of the AAV8 capsid in a manner completely distinct from the previously discovered AAVR-AAV interaction. In addition to showing the detailed interaction between AAVR2 and AAV8 (panel 2C), this figure also compares the differences between how AAVR and AAVR2 bind to AAV (panel 2D).
Future implications
This study reveals a previously unknown AAV transduction route that is independent of AAVR and varies by serotype. It underscores the importance of understanding the molecular determinants of viral entry and highlights the potential to optimize AAV gene therapy via receptor modulation. By demonstrating how certain AAVs can bypass canonical receptors, the research lays the groundwork for developing tailored vectors, expanding delivery options to tissues previously difficult to target.
*This article is featured in PKU News "Why It Matters" series. More from this series.
Read more: https://doi.org/10.1016/j.cell.2025.06.026
Written by: Akaash Babar
Edited by: Chen Shizhuo
Source: PKU News (
Chinese)

